SGLT2 Inhibitors and Healthy Aging
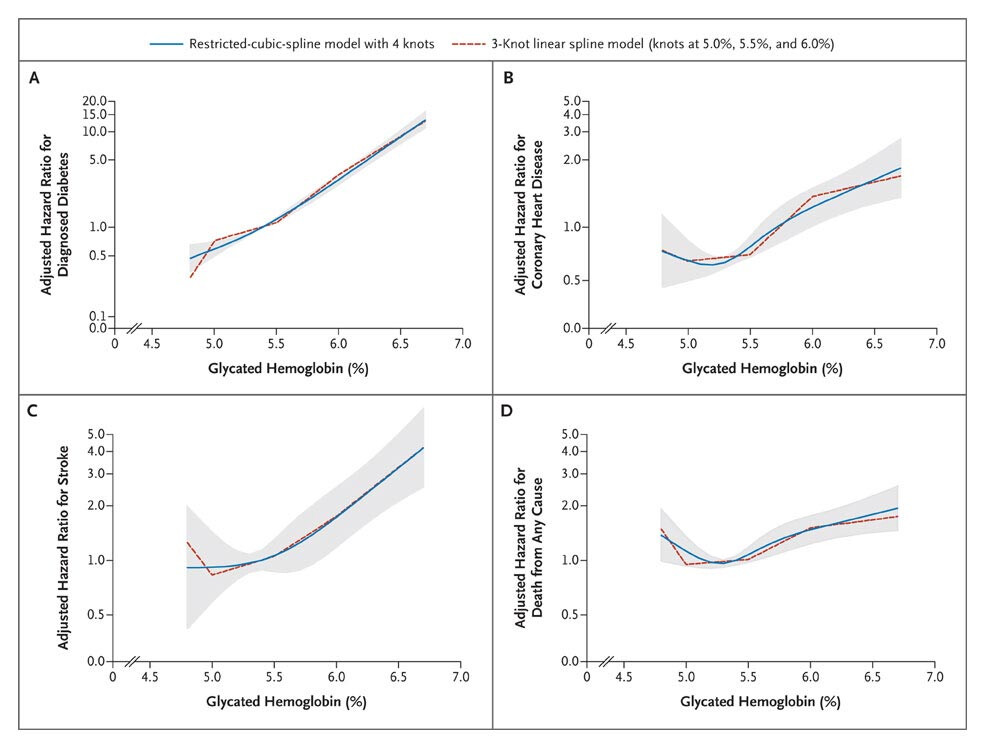
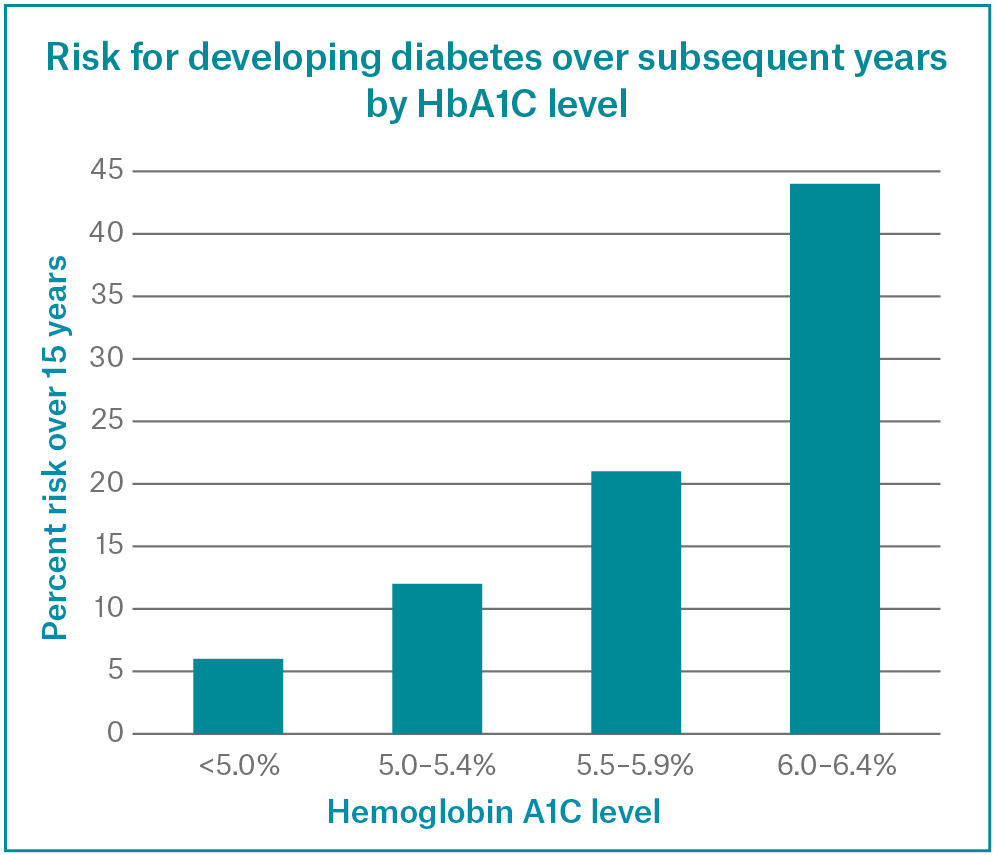
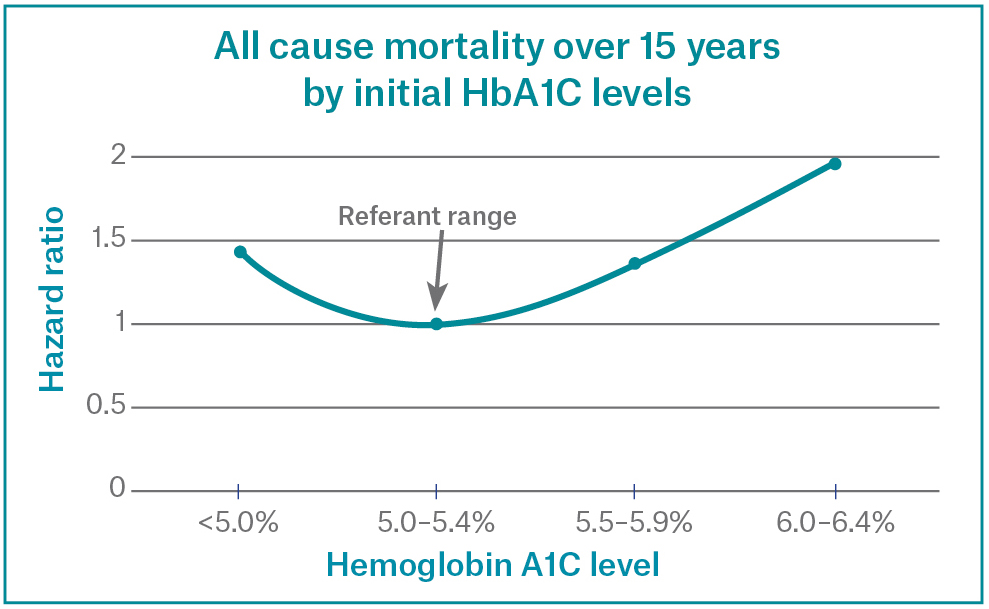
Recent research has shown some more interesting data about blood sugar and chronic ilness, even though the normal range according to most swiss labs and guidelines represents a range between 4.5% – 5.7% though when one looks at the data, it is clear that in healthy cohorts, especially in regards to metabolic longevity it is better to be in the lower part of this range. Of course caloric restriction and glycemic control represent the pillars of the hba1c optimisation, yet I wanted to talk about Sodium-glucose cotransporter‑2 inhibitors (SGLT-2i) for this purpose.
SGLTi promote urinary glucose excretion and induce a metabolic fasting–like state. An average dose shunts ~200 kcal/day of glucose into the urine leading to slight weight loss, sustained hyperglucagonemia and enhanced fat oxidation. These changes closely resemble caloric restriction (CR): blood insulin and IGF-1 levels fall, while nutrient-sensing kinases (AMPK, SIRT1) are activated and mTORC1 signaling is suppressed. For example, in non-diabetic mice canagliflozin increased blood β-hydroxybutyrate and upregulated AMPK/SIRT pathways, thereby mimicking CR and improving metabolic markers. These shifts trigger downstream effects that intersect canonical longevity pathways. Taking SGLTi for a healthy person though will not cause any dramatic changes, the most dramatic change one will notice is increased thirst and urination, which gets better after a week.
Animal Evidence for Longevity and Healthspan
Mouse studies provide compelling proof-of-concept that SGLT2 inhibition can extend lifespan. In genetically heterogeneous, non-diabetic mice, long-term canagliflozin treatment (starting at middle age) increased median lifespan ~14% and 90th percentile age by ~9% in males. This longevity benefit was accompanied by a lower incidence of age-related pathologies (cardiomyopathy, nephropathy, arteriosclerosis, hepatic steatosis, adrenal neoplasms) in treated animals. However, effects were sex-specific: female mice showed no lifespan gain or even slight reduction in some studies. In very old (30-month) male mice, late-start canagliflozin still improved activity and frailty metrics. Another recent study showed that empagliflozin improved lifespan and reversed liver senescence in naturally aged mice. These data indicate that SGLT2 inhibitors can act on fundamental aging processes in healthy animals.
Consistent with lifespan data, SGLT2 inhibitors also improved “healthspan” endpoints in models of aging or metabolic stress. For example, in diet-induced obese mice (without frank diabetes), canagliflozin reduced visceral fat inflammation, improved insulin sensitivity, and lowered senescence markers in adipose tissue. In progeroid (premature aging) mouse models, canagliflozin extended survival even when treatment began late. A variety of preclinical studies link SGLT2i to improved mitochondrial function, reduced oxidative stress, and normalized metabolic flexibility – all features of healthier aging tissues. Importantly, these benefits often exceeded those of pure glycemic control: in obese mice, insulin therapy normalized blood glucose but did not reduce senescent-cell load, whereas canagliflozin did. This suggests longevity benefits derive from non‑glycemic mechanisms (e.g. nutrient sensing and immune effects).
Blood Glucose Regulation in Non-Diabetics
In people without diabetes, SGLT2 inhibitors still induce modest glucosuria but have a limited effect on normoglycemia. The extent of glucose loss is proportional to filtered load: the higher the blood glucose (and GFR), the more is excreted. Thus, in normoglycemic subjects the net glycemic drop is small. For example, the diabetologia review notes that “the glucose-lowering effects of SGLT2 inhibitors are attenuated in individuals without diabetes”. Nevertheless, even in healthy individuals a full-dose SGLT2i can induce significant post-meal glucose alterations. In a controlled trial of healthy volunteers, a single 300 mg dose of canagliflozin increased 6‑hour urinary glucose excretion (~18 g) and reduced 0–2 h postprandial glucose by ~35% (and insulin by ~43%) compared to placebo. This effect arose from two mechanisms: renal glucosuria and a delay in intestinal glucose absorption. The latter occurs because high luminal canagliflozin partially inhibits SGLT1 in the proximal gut, blunting and spreading out glucose entry. A similar contrast was seen in a head‑to‑head study: canagliflozin (300 mg) produced more glycosuria and smaller postprandial glucose spikes than dapagliflozin (10 mg) in healthy subjects. In sum, SGLT2i modestly lower fasting glucose even in non-diabetics, and canagliflozin uniquely attenuates post-meal excursions via its partial SGLT1 block.
Importantly, these effects occur without causing hypoglycemia in normal subjects. Since SGLT2i activity diminishes as glucose falls, the drugs self-limit their glucose reduction. (In contrast, insulin or sulfonylureas can drive hypoglycemia regardless of ambient glucose.) Nonetheless, clinicians note increased glucosuria even at euglycemia can slightly increase thirst and urine volume in healthy users, which normally plateaus after a few weeks. Any sustained ketogenic effect is mild: BHB may rise slightly but rarely above levels seen in fasting/keto diets. Thus, in non-diabetics SGLT2 inhibitors offer a “fast-like” metabolic shift with only minor decrements in normal glucose levels.
Cardiometabolic and Renal Protective Mechanisms
Beyond blood sugar, SGLT2 inhibitors engage multiple cardiometabolic axes that could promote longevity. In large trials they markedly reduced heart failure events, progression of kidney disease, and even all-cause mortality – effects seen even in non-diabetic patients with heart or kidney disease. Mechanistically, these benefits arise from hemodynamic and cellular effects: SGLT2i induce mild diuresis/natriuresis (offloading the heart and kidneys), lower blood pressure, reduce arterial stiffness, and enhance endothelial function. For instance, dapagliflozin and empagliflozin restore nitric oxide bioavailability and improve vasodilation under inflammatory conditions. They also inhibit cardiac Na^+/H^+ exchanger (reducing cytosolic Na^+) and limit cardiomyocyte stress in failing hearts. In the kidney, reduced glomerular hyperfiltration and lowered intraglomerular pressure slow nephropathy. SGLT2i also trigger favorable fuel shifts in heart and muscle toward ketone use, which may improve cardiac efficiency. All these effects alleviate chronic organ stress and are independent of blood glucose. Long-term, this means SGLT2 inhibitors could delay common age-related diseases – e.g. they consistently cut new-onset diabetes, heart failure hospitalizations, and progression of fatty liver. In turn, reducing these pathologies indirectly extends healthy lifespan in populations.
Unique Features of Canagliflozin
Canagliflozin differs pharmacologically from other gliflozins in a few key ways:
- Off-target SGLT1 Inhibition: Canagliflozin is the least selective for SGLT2 over SGLT1. In vitro its SGLT2:SGLT1 selectivity is only ~155-fold (versus ~1200–2500× for empagliflozin/dapagliflozin). Clinically, a 300 mg dose of canagliflozin can partially block intestinal SGLT1 for a few hours post-dose. This slows glucose uptake in the upper gut, blunting postprandial spikes. In T2D patients, canagliflozin monotherapy increased post-meal GLP‑1 and decreased GIP, effects attributed to delayed glucose absorption. Such incretin changes are not seen with more selective SGLT2i. Thus canagliflozin may offer extra postprandial glucose control and possibly augmented GLP-1 signaling, which could have ancillary metabolic benefits.
- Pharmacokinetics: Canagliflozin is highly protein-bound (~99%) and has a half-life of ~10–13 hours (supporting once-daily dosing). By contrast, dapagliflozin and empagliflozin are ~90% and ~86% protein-bound with similar 12–13 h half-lives. Canagliflozin’s Tmax (peak) is ~1–2 h fasting (food slows absorption). Its large volume of distribution and extensive PPB may prolong tissue exposure. These PK differences mean that at equivalent doses canagliflozin achieves higher early plasma levels, contributing to its more pronounced renal threshold lowering and UGE.
- Glucose-Lowering Potency: In head-to-head studies with healthy subjects, canagliflozin 300 mg produced greater urinary glucose excretion (UGE) and larger reductions in the renal threshold for glucose, compared to dapagliflozin 10 mg. This translated into smaller postprandial glucose excursions with canagliflozin. Thus canagliflozin (especially at its higher 300 mg dose) often shows modestly stronger HbA1c and fasting glucose lowering than the others. Part of this potency likely stems from SGLT1 action and possibly mild AMPK activation, although no direct outcome trials compare gliflozins.
- Safety Signals: Canagliflozin’s distinct profile comes with some unique risks. In the CANVAS trial (Cana Study of the CANagliflozin Effect on cardiovascular events), canagliflozin was associated with a modestly higher risk of lower-limb amputations (2.6-fold) and bone fractures. These signals were not seen with empagliflozin or dapagliflozin in their major trials. The mechanism is unclear; hypotheses include volume depletion affecting peripheral perfusion. Clinicians remain alert to these issues when using canagliflozin, especially in at-risk patients.
Pharmacological Profiles of Select SGLT2 Inhibitors
| Property | Canagliflozin (InvoKana) | Dapagliflozin (Farxiga) | Empagliflozin (Jardiance) |
|---|---|---|---|
| SGLT2:SGLT1 Selectivity | ~155× (less selective; partial SGLT1 block at 300 mg) | ~1200× (highly selective for SGLT2) | ~2500× (highest SGLT2 specificity) |
| Protein Binding | ~99% | ~91% | ~86% |
| Half-life (t<sub>1/2</sub>) | ~10–13 hours | ~12–13 hours | ~12 hours |
| Dosing (T2DM) | 100–300 mg once daily | 5–10 mg once daily | 10–25 mg once daily |
| Renal Threshold for Glucose (RT<sub>G</sub>) | ~3.9 mmol/L on 300 mg | ~4.5 mmol/L on 10 mg | ~4.5 mmol/L on 10 mg (approx.) |
| UGE at Fasting Glucose ~9 mmol/L | ~60–80 g/day (higher UGE) | ~40–50 g/day (at 10 mg) | ~50–60 g/day (at 10 mg) |
| Notable off-target effects | Partial SGLT1 block (gut), ↑GLP-1 | Minimal SGLT1 inhibition | Minimal SGLT1 inhibition |
| GLP-1/GIP effect | ↑ Postprandial GLP-1 (delayed absorption) | ≈ no effect | ≈ no effect |
Table: Key pharmacologic parameters of selected SGLT2 inhibitors. Data from product information and clinical studies.
Cardiometabolic and Renal Benefits (Non-Glycemic)
Even in non-diabetics, SGLT2 inhibitors confer significant cardiovascular and renal protection, likely through glucose-independent mechanisms. Large trials in heart failure (HFrEF and HFpEF) and chronic kidney disease have shown reductions in hospitalization and mortality regardless of diabetes status. Proposed mechanisms include: decreased preload/afterload via natriuresis; improved myocardial energetics by shifting to ketones and free fatty acids; reduced fibrosis and hypertrophy (AMPK‑mediated); and attenuation of adverse kidney hyperfiltration. These broad effects – many overlapping with CR benefits – should logically translate into healthier aging organs. In fact, a meta-analysis shows SGLT2i lower new-onset diabetes incidence in CKD/HF patients, suggesting improved systemic insulin sensitivity or β-cell stress relief. Altogether, by preventing or delaying heart disease, kidney failure, and metabolic syndrome, SGLT2 inhibitors act as disease-modifying agents.
Safety and Long-Term Risks in Healthy Users
Data on very long-term SGLT2i use in healthy people are limited. Known class risks (gleaned mostly from diabetics) include genitourinary infections (from glucosuria), volume depletion/hypotension, and rare euglycemic ketoacidosis. For non-diabetics, DKA is uncommon unless additional stressors (very low-carb diet, illness) are present. A transient drop in blood pressure or orthostatic symptoms can occur in elderly users due to mild diuresis. Canagliflozin-specific concerns include bone fractures and amputations seen in diabetic trials; whether these translate to non-diabetic users is unknown but warrants caution. Claims of bladder cancer risk have not been substantiated. Overall, apart from more frequent yeast and urinary tract infections due to glycosuria, the safety profile in normoglycemic individuals appears acceptable. Notably, preliminary reports even suggest a lower incidence or severity of pneumonia with SGLT2i use, possibly reflecting enhanced immune health – though this finding needs confirmation. Ongoing surveillance and trials will clarify the net benefits and risks of SGLT2 inhibitors as longevity therapies.
Sources:
- Ferrannini E, et al. Mechanisms of action of SGLT2 inhibitors and their role in the metabolic paradigm shift. Diabetes Obes Metab. 2014;16(5):433-442.
- Miller RA, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(7):e140019.
- Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16(6):317-336.
- Powell DR, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, improves glycemic control in patients with type 1 diabetes. Diabetes Care. 2021;44(2):432-440.
- Neal B, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657.
- Zelniker TA, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis. Lancet. 2019;393:31-39.
- Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71(5):471-476.
- van Bommel EJ, et al. SGLT2 inhibition in the diabetic kidney—from mechanisms to clinical outcome. N Engl J Med. 2019;381(7):684-687.
- Polidori D, et al. Canagliflozin, but not dapagliflozin or empagliflozin, lowers postprandial glucose via SGLT1 inhibition in healthy subjects. Diabetes Obes Metab. 2013;15(7):661-668.
- Cahn A, et al. Clinical pharmacology and therapeutic potential of SGLT2 and SGLT1/2 inhibitors in type 2 diabetes. Diabetes Ther. 2020;11(9):2021-2039.
- FDA Invokana (canagliflozin) prescribing information.
12